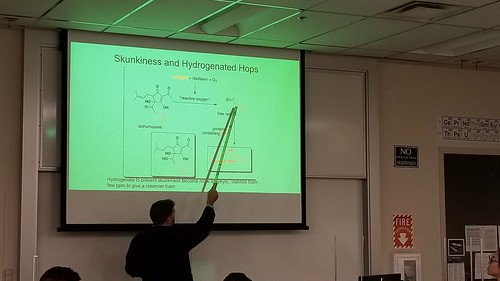
Max Mahoney (Chemistry) and I spent a long day brewing a couple of batches of beer recently. Now familiar with the process and how to execute it in the makerspace, we were able to add lots of variables and processes and gear, building on our experience with Brew Day v2.0, and our original, somewhat less-than-successful Brew Day v1.0.
One of the biggest changes to the process this time around had to do with the water. Specifically, we started with deinonized (DI) water, and Max added various salts to create ideal water, which as I understand it is a big factor in the overall success of the end product.
We brewed two batches of beer, and one of kombucha, which we’re fermenting in one of the smaller fermenters.
Testing the pH using one of the new meters.
Improvised wort chiller, until our conterflow setup arrives.
Into the fermenters.
We documented things more completely throughout the process this time. Timing notes are especially important, as we’re still working out how these brew days work as labs and activities. How many simultaneous batches make sense, and how many people can effectively work in the space at the same time.
Meanwhile, batch the second looks and tastes pretty good, with some distinct and pleasant apricot notes.
We’ve got both fermenters in The Spider Shed (a former chemical storage building that we’re repurposing as a nanobrewery) hooked up to a heating and cooling system, and they’re happily bubbling away.
We’re still learning so much, and the process is becoming even more interesting. Max is especially stoked about tweaking the water chemistry, and has lots of ideas about wrapping class activities and labs around that process. A couple more days, and we’ll cold crash these batches and keg ’em up!